Developing a New Medicine Takes a Third of a Scientist’s Career
 On average, around 50 brand-new medicines are added to the pharmaceutical market each year. Why so few? Because it can take as long as 10-15 years to develop and research a new medicine. This is about 1/3 of a scientist’s career. In addition, the cost of developing and testing a medication before it reaches the market costs around €950 million ($1 billion). This is equivalent to employing 1,293 scientists in Lithuania for 30 years at an annual salary of around €24,500.
On average, around 50 brand-new medicines are added to the pharmaceutical market each year. Why so few? Because it can take as long as 10-15 years to develop and research a new medicine. This is about 1/3 of a scientist’s career. In addition, the cost of developing and testing a medication before it reaches the market costs around €950 million ($1 billion). This is equivalent to employing 1,293 scientists in Lithuania for 30 years at an annual salary of around €24,500.
The initial stage of this costly and time-consuming process starts in the laboratory. The production of proteins linked to certain diseases and searching for the candidate drugs to inhibit them is also underway at Vilnius University’s Life Sciences Centre. According to Dr Joana Smirnovienė, a researcher at the Centre, the Laboratory of the Biothermodynamics and Drug Discovery Unit is using advanced biochemical and biophysical methods to identify the most promising chemical compounds, which could be used in the future to diagnose and treat various diseases. Dr Smirnovienė and her team are currently working to develop new cancer drugs.
Researching proteins associated with different diseases
How do scientists learn about the need for a new medicine? According to Dr Smirnovienė, on the one hand, the need comes from consumers (or the pharmaceutical companies) when they notice that the existing drugs for the disease are not effective enough, are expensive or have too many side effects. There is also a growing trend for pharmaceutical companies to look to scientific laboratories or start-ups with relevant research and buy the developed technologies from them. Of course, research into new drugs for more common diseases is more likely to receive funding than research into drugs for rare diseases.
According to the researcher, developing modern medicines in the laboratory starts with identifying the proteins involved in the disease and finding chemical compounds to target those particular proteins. For scientists to start their research, they first need to choose the proteins they will work with and make them identical to those found in the human body. Some proteins can be extracted from human saliva, while others are obtained from blood or mammalian cells. Scientists can even use bacteria to produce a protein analogous to one that exists in the human body, which can then be used for research.
“For a long time, our lab team has been studying a group of proteins that is made up of 12 members, which are very similar to each other but are each linked to a different disease (e.g. glaucoma, obesity and cancer). Among these family members are several proteins that are essential for the human body. Therefore, our aim is to find the minuscule differences between these family members because we need to make sure that the drug inhibits only the one member of the protein family that is exclusively found in cancer cells and is involved in tumour development, but does not affect the remaining 11,” the scientist explained.
The disease-causing protein has its own activity, which is linked to the development of the disease. That’s why the researchers at the Life Sciences Centre are looking for chemicals that can inhibit the activity of a specific cancer-related protein to stop the disease from progressing.
Testing the effectiveness of a new cancer drug
Once the proteins to be worked on have been selected and tested, the most effective chemical compounds – drug candidates that inhibit the activity of the disease protein – are searched for and screened to see how they interact with the protein produced in the lab. The search for potential medicines is based on naturally occurring chemical compounds or on attempts to develop new substances using chemical synthesis, computer modelling and crystallography.
“We have discovered several effective chemical compounds, including some that interact exclusively with a protein that is present only in cancer cells. Our discovery has attracted the interest of American scientists, who are continuing their research on these compounds. They have attached a luminous tag (which glows under a specific light) to the molecules we discovered and used it as a carrier. It can then be used in cancer surgery, where the cancer cells need to be isolated and identified to allow for a precise excision of the tumour,” explained the young researcher.
Furthermore, the scientists at Vilnius University are now conducting pre-clinical studies of this drug candidate and are testing its efficiency in mice. Mice with cancer are injected with a candidate drug, and the size of the tumour is then measured and monitored for shrinkage, while the test subjects are compared with other mice that have not been administered the drug.
In pre-clinical studies, testing can be performed either in animals (in vivo) or on cells from humans or animals in a Petri dish (in vitro). However, in vivo studies are often preferred, as they allow the effects to be monitored in the whole living organism rather than just in isolated cells.
Only 2% of drug candidates are tested in humans
At the start of the development of a new drug, around 10,000 chemical compounds are synthesised, from which around 250 drug candidates are selected to go into a second phase of research where they undergo pre-clinical studies. Only 5 of these 250 drug candidates will go on to be tested in humans, and only one in five successfully progresses to the clinical trials stage before being approved (Figure 1). 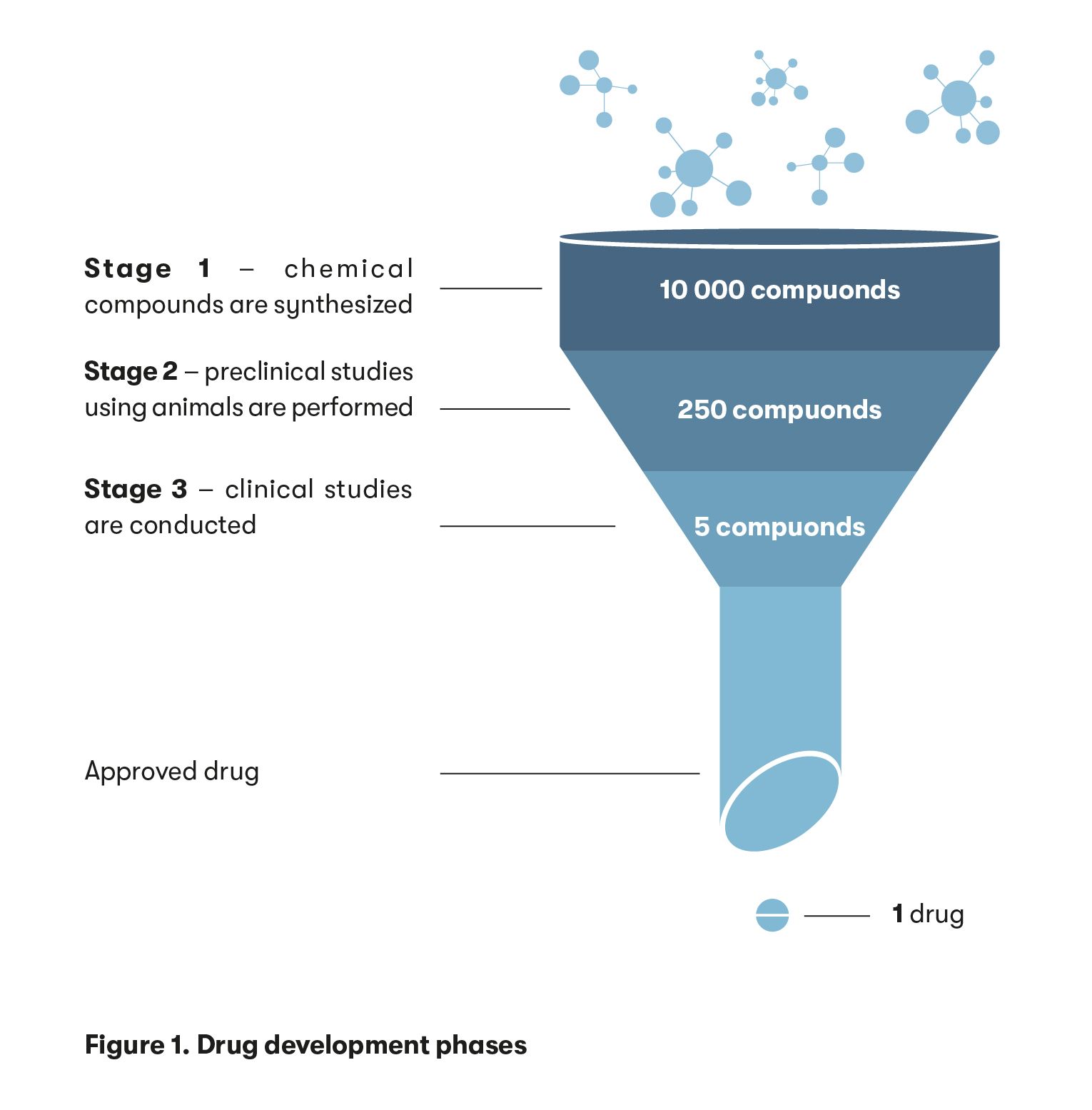
“Our laboratory has synthesised over a thousand chemical compounds, but only five have made it to the pre-clinical stage. Typically, hundreds of thousands of compounds are tested in laboratories around the world. Only around ten of them make it to the next stage of research – animal testing – and only a few make it to human clinical trials. Nonetheless, we have to synthesise those hundreds of thousands of compounds to select the most suitable ones,” said Dr Smirnovienė.
In the first phase of a clinical trial, the scientist explained, the drug is tested for its safety in healthy volunteers. In the second phase, small groups of 10-100 people with a particular disease will receive the medicine. They are administered a safe dose of the drug and are monitored for side effects. If all goes well, the drug is then tested with a larger (up to 100,000) group of people, divided into healthy and unwell individuals, who receive either the drug or a placebo. They don’t know which they have been administered – the drug or a placebo. The data from this study then determines whether the medicine will be validated and registered with the European Medicines Agency (EMA).
If such a study shows that the benefits of a medicine outweigh the harm (e.g. only one in 100,000 will experience a side effect, or more than 70 % of the subjects will benefit from the medicine), it may be placed on the market. The data from this study, which can last up to eight years, also determines whether the medicine will be legalised. An application is then submitted to the EMA with all the necessary information to register the new medicinal product. On average, it takes another year and a half to verify this information.
However, the pandemic has shown that the development of a new drug or vaccine can take as little as two years, provided that the necessary financial and human resources are mobilised and that a large amount of scientific knowledge is accumulated on coronaviruses, which had already been studied by scientists for a decade prior to the epidemic.
Adapting the side effects to treat other diseases
Despite the lengthy, painstaking and strictly regulated animal and human studies of new drugs, scientists and medical professionals are increasingly aware that diseases develop differently in each person. The heterogeneity of the patient population means that certain medicines may only be effective in certain patients, while in others, they may cause severe side effects.
“There have been cases in the past where, for example, an arthritis drug on the market was beneficial and generated billions of dollars of revenue for Merck, but when it was found to increase the risk of myocardial infarction and stroke, it had to be stopped because it caused too much harm,” recalled the scientist.
However, the opposite is also true, where the side effect of a medicine is discovered in pre-clinical or clinical trials, and it is found that the same medicine can be used to treat another disease. For example, two years ago, a team of researchers at Goethe University Frankfurt found that an anti-diarrheal drug (loperamide hydrochloride) can cause the death of specific cells, which could be used in the treatment of a rare but aggressive and deadly form of brain cancer called “glioblastoma”.
“Clinical trials of a drug for epilepsy (topiramate) have shown that it has the side-effect of causing weight loss, so it could also be used to treat another condition – namely, obesity. The drug molecule has a structural element that allows it to bind to a family of proteins that we are studying in our lab. We found that this drug interacts particularly strongly with one of the 12 members of the target protein family that could be responsible for obesity. My colleagues at the Life Sciences Centre are investigating how the same principle of COVID-19 RNA vaccine technology could be used to personalise the treatment of cancer and other diseases by replacing the informational RNA that tells the body which disease-associated protein to produce,” explained the biotechnologist.
A successful first stage is key
The time, complexity and cost of developing a drug are projected to decrease due to the rapid development of modern automated biochemical and biophysical methods, as well as advances in artificial intelligence and the accumulation of valuable scientific knowledge.
“To speed up and lower the cost of the drug development process, we analyse the mechanisms by which a disease-associated protein recognises a drug candidate at the molecular level. It is essential to select the most suitable molecule for further pre-clinical and clinical trials at the initial stage of drug development (while it is still in the test tube). If the wrong molecule enters the further stages and is tested, a huge amount of money and time is lost,” Dr Smirnovienė noted.