Scientist Dr. Algirdas Toleikis on What Molecular Motors Are and Why We Need Them
Imagine a city with a lot of things to transport. A product is produced in one place and needed in another. In a city, goods are transported by trucks. Cells are also like a big busy microscopic city where intracellular vesicles with proteins, hormones, neurotransmitters etc. are transported. Only here, the transport function is carried out by molecular motors, of which there are thousands in each of our cells. “Every car in a city is driven by a human being, they have a driving licence, they obey the traffic rules, they know what cargo they are carrying and where it needs to be delivered. But who “drives” the molecular motors?” asks Dr. Algirdas Toleikis, a biochemist and biophysicist who studies what happens in our microscopic cities.
Dr. A. Toleikis established his laboratory in Lithuania a few years ago, after nine years of his research career in the United Kingdom, where he went after winning a prestigious Wellcome Trust scholarship. Although he had a brief stint as a programmer, he returned to research with a grant from the European Molecular Biology Organisation (EMBO) to set up his own laboratory.
After years of studying what “drives” these molecular motors, Dr. A. Toleikis is now trying to understand how DNA motors work, which in some cases may in the future be more efficient than gene scissors. We spoke to Dr. A. Toleikis about this at his workplace, Vilnius University Life Sciences Center.
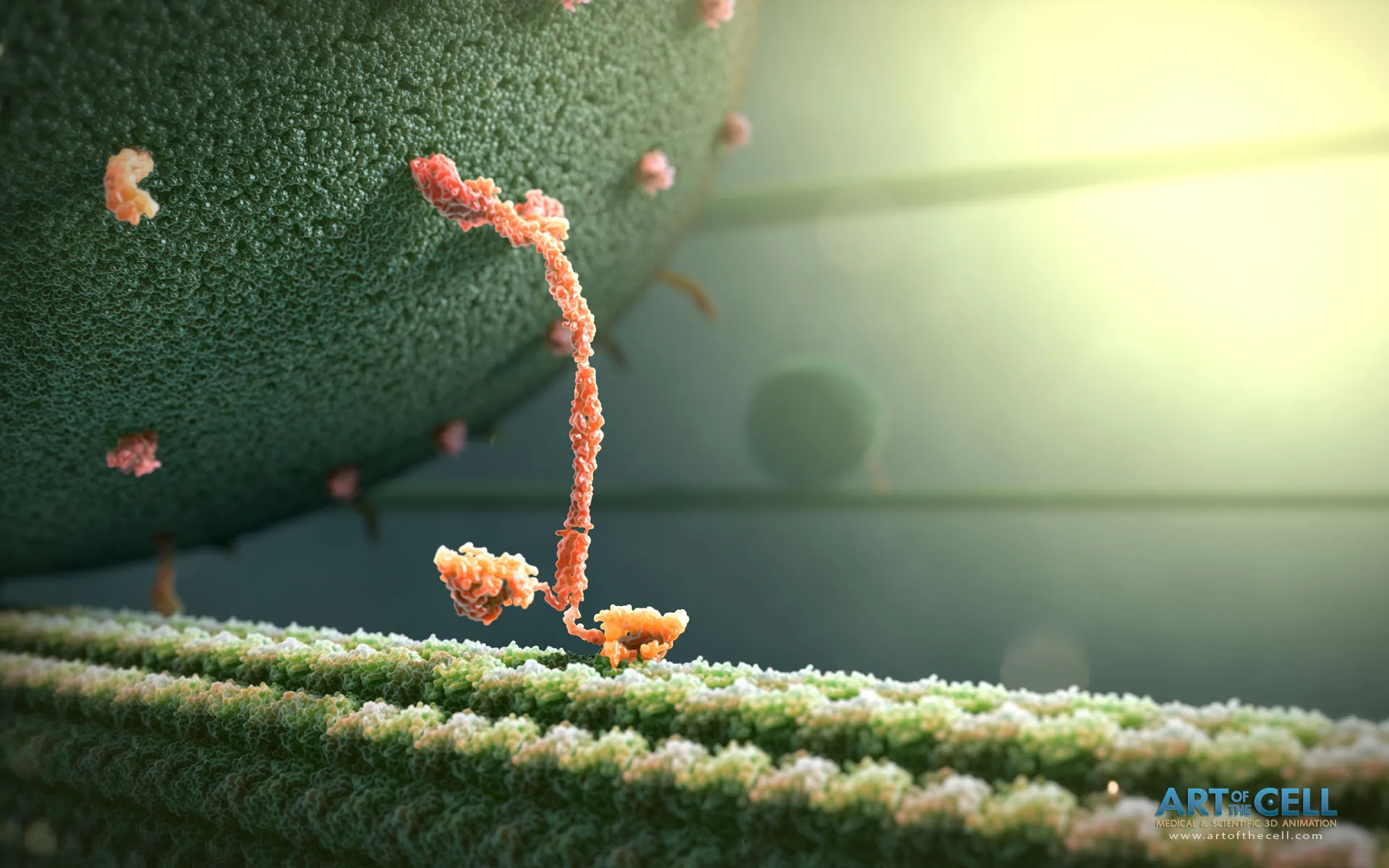
“Kinesin is one of the best-recognised molecular motors. It was one of the first to be discovered, almost 40 years ago. It is also an interesting story! Kinesin was discovered by Dr. Ron Vale, an American biochemist, when he was still a student. His postdoctoral supervisor tried to purify molecular motors from the tentacles of an octopus but was unsuccessful and put the experiment aside. Vale came and conducted one more experiment and saw something moving under the microscope. It turned out to be a kinesin”, the researcher begins the conversation.
As I understand it, kinesin is the best-studied molecular motor?
There are many molecular motors - many different families of different molecular motors. There are thousands of them in a single cell.
To understand them better, we need to take one molecular motor as a model. Usually, the best-studied motor is taken as the kinesin, but there are 15 families of kinesins alone, and within each family, there are subfamilies etc. That’s a lot! But the one that is most often studied is the one that is more familiar, that behaves well, like a pet (laughs).
Your postdoctoral studies focused on kinesin research. What were you trying to learn? And what did you learn?
Yes, I chose the University of Warwick for my postdoc. I was very interested to see how kinesins work.
Imagine a city. A lot of things have to be transported. In one site, a product is produced, but it is needed in another site. In a city, goods are transported by trucks. Cells are like a big and busy city, but microscopic - they transport intracellular vesicles containing proteins, hormones, neurotransmitters etc. Molecular motors also come in all sorts of forms - if kinesins are trucks, other motors are tractors and passenger cars.
There are some very nice videos, created by using glowing dyes targeting kinesins, showing how molecular motors transport proteins in the cell. It looks like a city at night from an aeroplane.
What I found most interesting is that if every car in a city is driven by a human being, they have a driving licence, they obey the road rules, they know what cargo they are carrying and where it needs to be delivered. And who “is driving” molecular motors? How do these kinesins know everything? How can they do it?
And is there any consideration on what regulates that transport process? In other words, what “drives” the kinesins, for example?
It is a very complex question. However, I think we should start from the fact that at the beginning we did not even know how a kinesin moves. Now we have videos that have been made where we can see that the kinesin is sort of moving, step by step. There has been a lot of research to establish that, and we are still not 100% sure how it works.
I wanted to find out how the kinesin moves. It was already known that kinesin sometimes goes backwards. So for 2–3 years, I have been studying how the kinesin ‘steps’, what happens when it stops and when it can’t go anymore, and how it works in ‘reverse gear’. For example, when we go backwards, we can do it in a couple of different ways: we produce a kind of mirror image of going forward, or we turn around and just go backwards. It was not clear how the kinesins go backwards. What we have shown is that the kinesins walk like Michael Jackson in his branded moonwalk dance (laughs). It’s like they’re sliding or skating backwards.
How do researchers know that kinesin slides instead of raising its “legs”?
We have made many observations using a technique called optical tweezers, which allows us to “catch” a single molecule of kinesin. For their development and application to study molecular motors, Arthur Ashkin was awarded the Nobel Prize in Physics in 2018. It involves shining a highly focused optical laser beam at the kinesin, which is attached small polystyrene microsphere, which you can use as a handle to pull on the kinesin and monitor its movement in real-time. In other words, we can use optical tweezers to control the kinesin.
I have spent many hours doing this, watching many thousands of steps. When the kinesin steps, how much and when it needs to be pulled, when it would stop, when it would go backwards or fall off completely etc. From these data, we realised that all the other hypotheses could be rejected, and the only logical one was that it was not stepping backwards, but rather sliding.
What is there in the kinesin cargo?
There could be proteins, hormones, neurotransmitters etc. This transportation takes place in virtually every human cell. However, the most intense action is in brain cells neurons. For example, there are motor neurons: it can be up to a metre in length. The cell itself is very small, but the projection of the neuron - the axon - extends from the spinal cord to the fingertips. It is a projection of a single cell. Imagine, at the end of the axon, a protein or neurotransmitter is needed, which is produced in the cell body in the spinal cord. For kinesin, this journey takes about two weeks. But if kinesin were not present, the required protein would never get there by passive diffusion.
Can humans replace the cargo of molecular motors? For example, using genetic engineering?
This could be the technology of the future. What we need now is to understand how kinesins and other molecular motors work, and how to fix them if they don’t. For example, if kinesins break down, a person develops a neurodegenerative disease like amyotrophic lateral sclerosis (ALS); molecular transport is even linked to Alzheimer’s disease etc. Therefore, if we understand why molecular transport is broken in the cell, in principle, we could fix it and cure the disease. There is no such treatment yet, but it is possible in the future. I believe it could be as soon as 20 years from now.
In addition, with a better understanding, we could probably find more applications in innovative medicine. Not necessarily where kinesin repair is needed. For example, to transport drugs to a specific site in the cell.
In Lithuania, you have started a new direction: research on DNA motors. Tell us what it is.
Whereas kinesins are urban freight transport, DNA motors are like construction site machines, like bulldozers. DNA motors also need energy, they also move, but not like kinesins, they usually move like a worm.
We need to understand them because they have many important functions related to DNA. For example, when you copy DNA during cell division, the DNA needs to be unwound first, because it consists of two strands. It is only then that the information can be copied. The DNA unwinding is carried out by the DNA motors helicases. Once we understand them, we will know how one of the most important processes in the cell - the copying of DNA - works. We could also apply them to gene editing. If we attached an instrument to a DNA motor – let’s call it a ‘mower’ - that would ‘cut down’ the unnecessary parts of DNA, even the big ones. You could even program the DNA motor to work only in a specific site.
A few years ago, you were awarded an EMBO Installation Grant (EMBO - European Molecular Biology Organisation) for the development of DNA motor research - you were the first person in Lithuania to receive it.
Yes, I received funding to set up my own independent research group focusing on DNA motor research. We will try not only to understand them better but also to find out how they can be applied in genome editing. This means how we can use DNA motors, also known as DNA shredders, rather than gene-editing scissors.
Gene editing DNA scissors have one major drawback: they can cut one or more letters out of the DNA sequence. However, if you need to cut out a single gene or a large fragment of the genome, scissors are not enough - you need a DNA shredder. It is necessary when we want to either switch off genes if they cause disease and are not necessary or if we want to understand what certain parts of the genome do. It is this DNA shredder that we are investigating. We have already bought the basic reagents and equipment, and we are building the microscopes for that ourselves.
You had a break from science, working as a programmer for an IT company. But you came back. Why did you decide to leave academia and eventually returned back?
When I decided that I wanted to come back to Lithuania, I was already finishing my postdoctoral research. Like every researcher at this stage, I kind of was at the end of the road - either you try to get funding for your lab, or you look for other postdocs, which doesn’t really help and only makes it harder to get funding, or you do something else.
Statistically, according to the data of the Royal Society in the UK, only about 1% of PhD graduates set up their own labs, which means a lot of competition, which is not to everyone’s liking. Even if you set up a lab, you have to constantly compete for funding. Nor does everyone like the fact that the head of the laboratory is moving from being a researcher to being more of a manager.
I also needed a break, I wanted to think and crystalize my ideas, and I didn’t want to just passively continue the same route. Just then, an IT company offered me a job because I was taking a course at a programming school. I really like programming, and it’s also very much needed in research work. So I wanted to learn how to program better, but I got a job in Lithuania (laughs). It was an adventure that turned out to be a very good decision in the end.
I learnt from professional programmers, in one of the most serious companies in Lithuania - I learnt things that I would never have learnt in academia. What is the absolute norm in business may be completely unknown in the academy (laughs). Now I can bring to the lab completely different industry standards and principles in both programming and project management. I am trying to introduce them in my lab as well. So breaking out of the academic bubble has been very useful for me.
Your older brother Zigmantas and sister Gabija are also researchers. Gabija has even become a writer and author of the book “Why the F*ck Can’t I Change?”. Did they influence you to become a researcher too?
Older siblings are always an influence. I saw the academic choices of Zigmantas and Gabija. And I can’t be left behind either! (laughs) So I really tried to keep up.
Gabija did her PhD in the UK because she got a Wellcome Trust scholarship, which is one of the most prestigious scholarships there. When I was thinking about what to do next after my undergraduate studies, I thought, hmm, if Gabija got it, I could do it too (laughs). In the UK, you can “skip” your Masters and go straight to a PhD. So I got the Wellcome Trust scholarship too. But I might not have thought about it if it wasn’t for my sister.
What do you like most about your work now?
I love programming and building research instruments. Now we can build a microscope that works! If you do it well, nobody else in the world has a microscope like that. There may even be specific questions that can only be answered with the microscope we have built. Since others don’t have one, they won’t know the answers that we can find out.
I really enjoy working with students. Especially when they are so inquisitive and hardworking. You can pass on your knowledge, you can see how someone is developing, you can see their path.