Vilnius University Scientists’ Research on Bacterial Defense from Viruses Was Published in “Science”

From left to right: Irmantas Mogila, dr. Giedrė Tamulaitienė and dr. Gintautas Tamulaitis. VU photo
A team led by Research Professor Gintautas Tamulaitis from Vilnius University Life Sciences Center (VU LSC) revealed a novel protection mechanism in CRISPR-Cas antiviral defense system. The article “Ribosomal stalk-captured CARF-RelE ribonuclease inhibits translation following CRISPR signaling” was published in the journal Science.
CRISPR-Cas systems provide interference against invading foreign nucleic acids by different mechanisms. In bacteria, several different types of antiviral CRISPR-Cas systems are found, which differ in Cas proteins and their functions. Currently, the best-known Cas9 and Cas12 proteins are widely used as genome editing tools. A team of researchers led by Gintautas Tamulaitis is studying type III CRISPR-Cas10 systems, which combine three different enzymatic activities to defend against viral infection. When a virus attacks a bacterium, CRISPR-Cas10, similar to Cas9 or Cas12, destroys the viral DNA, and also cleaves mRNA, and viral transcripts. In the paper published in 2017 in Science, this team discovered that after the detection of viral transcripts inside the cell, CRISPR-Cas10 antiviral system starts synthesizing unique signaling molecules – cyclic oligoadenylates. These molecules then activate various additional effector CARF proteins.
The recent publication in Science by Mogila, Tamulaitiene et al. represents a continuation of the successful scientific research conducted by Gintautas Tamulaitis’ group. In this study, the Vilnius University researchers using bioinformatic analysis, biochemical, and structural studies characterized a novel family of effector proteins, named Cami1. They showed that when a virus attacks a bacterium, CRISPR-Cas10 signaling molecules activate Cami1 - a ribosome-dependent ribonuclease. “Activated Cami1 cleaves mRNAs that are involved in protein synthesis, thereby inhibiting cell growth. This allows the bacterium to save resources and prevents the production of viral proteins,” says Gintautas Tamulaitis.
Using X-ray structural analysis and cryo-electron microscopy (cryo-EM), researchers determined structures of both apo-Cami1 and the Cami1 complex with the protein synthesis machine - ribosome. Structural studies provided insights into how Cami1 can specifically cleave mRNA. It was shown that Cami1’s interaction with a specialized ribosome structure called the ribosomal stalk, is necessary for its entry into the protein synthesis center. “Interestingly, the same capture mechanism to bind the ribosome is used by plant antiviral proteins that also inactivate ribosomes. This discovery unveiled an additional layer of the CRISPR-Cas antiviral defense system and demonstrated a common antiviral strategy shared between eukaryotes and bacteria. Knowledge about our characterized Cami1 proteins will contribute to the development of new molecular tools in biotechnology and therapy,” says Gintautas Tamulaitis.
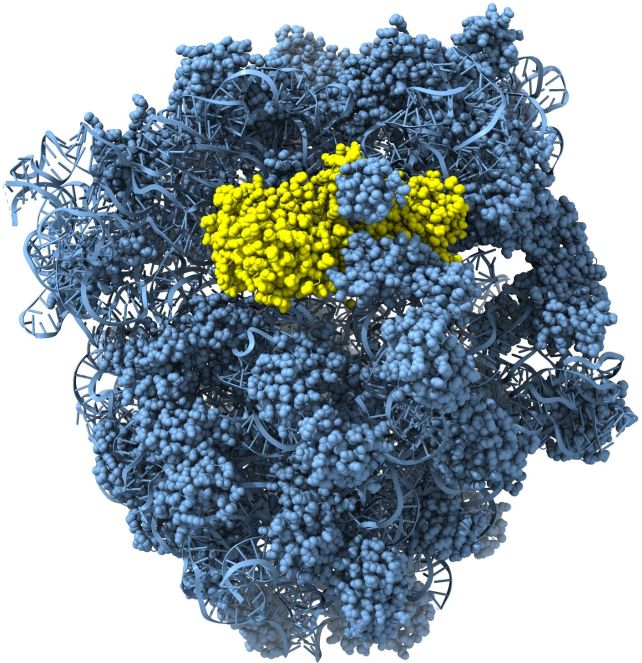
The ribosome-Cami1 complex was visualized using a 200 kV Glacios Cryo-Transmission Electron Microscope, acquired by Vilnius University in 2020. The first findings from this microscope were published in Nature earlier this year, and now, the next study has been published in Science.
The paper’s authors include PhD student Irmantas Mogila, Dr Giedre Tamulaitiene, master student Konstanty Keda, Dr. Albertas Timinskas, Audronė Rukšėnaitė, Dr. Giedrius Sasnauskas, Prof. Česlovas Venclovas, Prof. Virginijus Siksnys and Dr. Gintautas Tamulaitis.
Science is a prestigious, one of the most frequently cited international scientific journals that publishes top-reviewed research covering all fields of science and technology. The Science family of journals is published by the American Association for the Advancement of Science (AAAS), the world’s oldest and largest science organization.
This research was supported by the Research Council of Lithuania (grant S-MIP-22-09 to G. Tamulaitis) and Vilnius University (intramural grant MSF-JM-11 to I. Mogila).